The National Institutes of Health’s R01 grants are among the organization’s oldest and most prestigious awards presented to investigators conducting biomedical research. With only around a 20% success rate per application, receiving just one of these highly selective grants in a year, which provide support for up to five years, is a notable accomplishment for any faculty member. It is extremely rare to receive two R01 awards in the same year and is a feat that has not occurred at Syracuse University in the recent past.

Liviu Movileanu, professor of physics in the College of Arts and Sciences, was recently awarded a pair of R01 awards through the NIH’s Institute of Biomedical Imaging and Bioengineering and Institute of Medical Sciences totaling $3 million. The grants support his ongoing work to develop a generalizable nano-sized sensor capable of detecting proteins with high sensitivity and specificity. These nano-sensors could one day allow researchers to identify biomarkers for cancers and other diseases in complex biofluids.
These aren’t the first major NIH awards for Movileanu, who has been a professor at Syracuse since 2004. A $1.2 million grant resulted in a pivotal paper published earlier this year in Nature Communications, which formulated a nanopore sensor design architecture that can be applied to a broad range of protein targets.
To conceptualize a nanopore sensor, think of it like a “hook and bait.” A tiny protein binder acts as the hook and fuses to a small hole created in the membrane of a cell—known as a nanopore—which allows ionic solution to flow through it. When the sensor recognizes a targeted molecule, the ionic flow changes signaling that the biomarker has been found—like an angler hooking a fish.
Movileanu’s $1.4 million grant, titled “Development of Modular Synthetic Sensors for Protein Biomarker Detection,” will involve the development, optimization and validation of a next-generation class of sensing elements for targeted protein biomarker detection at single-recognition event precision.
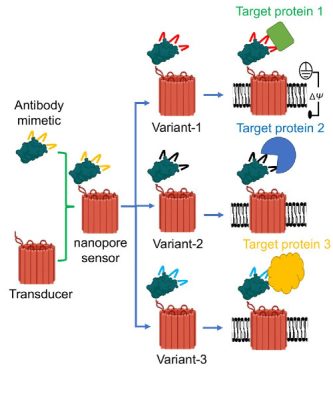
Proteins interact with each other on a one-on-one basis to carry out various jobs in the cell. When one protein “captures” another protein, it is known as a protein recognition event. Capture and release events of one protein by another are part of a reversible (transient) process within the cell that can take from a few microseconds to tens of seconds. When the interaction is too short or too long, it can result in perturbations in the chemical traffic inside the cell. Movileanu explains that through this grant, they hope to design tiny sensors to monitor these captures and releases in real-time and at the single-molecule level.
“We will utilize advanced protein engineering and single-molecule technologies to explore further advantages and shortcomings of nanopore sensors, yet with an emphasis on those chemicals that are amplified in the blood stream or other complex biofluids under disease-like conditions such as solid tumors or hematological malignancies,” says Movileanu.
To address upcoming fundamental challenges regarding detection of proteins at low numbers, the team will utilize a mechanism to amplify the sensor’s signal. The proposed design will also discriminate proteins with similar structures but different functional features, essentially weeding out protein look-alikes. Lastly, their study will identify subpopulations of similar proteins with different functional roles, which is challenging using existing technologies.
While the first grant is focused on developing a broad range of sensor structures, compositions, architectures and functions with a long-term goal of highly sensitive biomarker detection, the second $1.6 million grant will be exclusively focused on developing nanopore sensors for various classes of kinases, which offer strategic drug targets that have critical implications in numerous cancers. Titled “Generalizable Nanosensors for Probing Highly Specific Interactions of Protein Kinases,” this research will involve tuning nanopore sensors to identify and quantify a significant group of kinases.
Kinases are protein enzymes that facilitate a phosphate-transfer reaction known as phosphorylation, one of many mechanisms by which proteins are chemically modified after they are produced in the cell. Phosphorylation processes are involved in key cellular activities and functions, including cell growth, differentiation and cycle, as well as intracellular and intercellular signaling.
According to Movileanu, abnormal deviations in phosphorylation pathways result in profound molecular disturbances in signaling, ultimately leading to numerous cancers, diabetes and neurological diseases.
“Kinases are the most extensively studied molecular machines that regulate chemical modifications of proteins,” says Movileanu. “Because kinases are molecular machines at the heart of cellular functions, their functional features are critical under physiological and disease-like conditions.”
With this grant, the team will seek a better quantitative and mechanistic understanding of kinases which could uncover essential knowledge of how a disease state progresses through various phases. Their generalizable and highly specific nanosensors can also help address current challenges preventing the use of numerous kinase-targeted drugs already approved by the FDA.
In the future, Movileanu says this nanosensor technology may take the place of imaging and biopsies when diagnosing cancers. By integrating the sensors into nanofluidic devices, this machinery would allow scientists to test for many different biomarkers at once in a specimen, providing a fundamental basis for biomarker detection in complex biofluids such as blood.